Obtained PAJ-390 and PAJ-379 containing MG1655-Z1 cells from Professor Jaramillo’s lab.
Prepared plates with appropriate antibiotics for selection (spectinomycin for all Z1 cells, chloramphenicol for PAJ-390, kanamycin for PAJ-379).
Plated cells.
Cells have grown on the appropriate plates.
Prepared liquid cultures of both lines for miniprep.
Prepared liquid cultures of the 390-containing line for electrocompetence induction.
We induced competence in the cell line containing the 390 plasmid.
We miniprepped both plasmids – in hindsight the method was flawed as our yields were low.
First, we plated the SAJ-351 cells which contained the 390 (dCas9) plasmids. We also miniprepped the 379 (GFP) plasmid and obtained a concentration of 26.8 ng/µl.
Transformation failed, so we centrifuged a Falcon tube with the supposed transformants and resuspended the pellet in less media. We then plated the resulting concentrated culture
There was no growth from the transformants.
We tried to transform the SAJ-351 cells with the 379 plasmids. The 379 plasmid contained resistance to kanamycin (Kan), so if the culture grew on the Kan/Spec/Chlor plate then we would know the transformation had been successful.
Unfortunately, there was no growth, so the transformation was unsuccessful. However, the culture grew on the control plate without kanamycin, so the cells were not killed in the procedure and the error lied with either the transformation procedure, the concentration of the 379 plasmids, the competence of the cells or the 379 plasmid itself.
The control grew, but the transformants didn’t. Therefore we made more competent cells from our existing culture, but we forgot to add antibiotics so the procedure didn’t work.
We tried the transformation again but received the same results (no growth). As we had carefully followed the procedures for both transformation and competence, we decided the most likely cause for the lack of growth on the Kan/Spec/Chlor plate was either the 379 plasmid itself or the concentration of the 379 plasmids, as it was very low. Therefore, we re-did the 379 plasmid miniprep and received a concentration of 40.7 ng/µl.
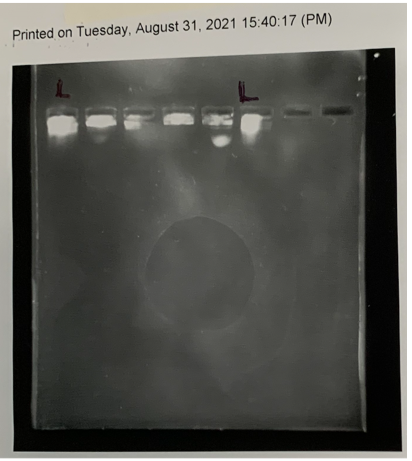
We also ran a restriction digest on the 379 plasmids to see whether there were any unexpected results. The results were inconclusive – after running the gel for an additional 40 minutes on top of the required 40 minutes in the procedure, there was still no visible ladder. We ran the gel again for another 20 minutes, but unfortunately the agarose melted which meant we had to try again another day.
The transformation failed again.
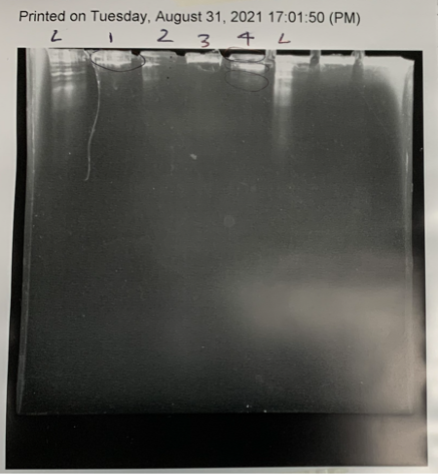
We repeated the restriction digest and it appears that it may have worked as we had somewhat of a visible ladder, but the integrity of the gel was weak, and it broke easily so we were not convinced of the results and planned to run it for a third time in the future.
We prepared new liquid cultures to repeat the miniprep.
We again repeated the transformation of the SAJ-351 cells, using the new miniprep of 379 plasmid. On the same day, we carried out yet another miniprep of the 379 plasmids to increase the yield to 242.7 ng/µl as a backup in case the transformation failed again.

However, the transformation was a success, creating a cell line which we called PAJ! A single colony grew on the Kan/Spec/Chlor plate, meaning it contained the plasmid with the kanamycin resistance gene. The success of the transformation meant we did not need to repeat the restriction digest of the 379 plasmid.
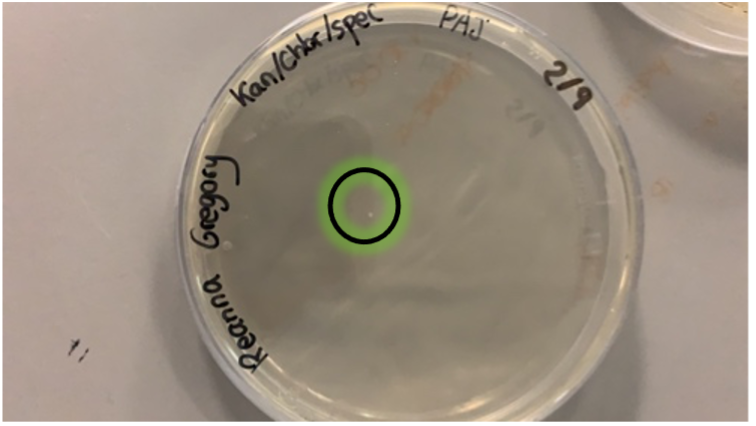
This single colony was replated on to obtain more of the PAJ cells, so we could use them to prepare liquid cultures and run different experiments.
Both cultures grew nicely, giving us confirmation that we had produced transformed cells.
We obtained the chemicals necessary for inducing the PAJ circuit – ATC and IPTG.
Our first attempt at PCR-extracting the backbone for the helper plasmid. This failed as we used the improper polymerase, ordered primers for extraction as 3 3 kbp fragments.
We also attempted our first run of the PAJ fluorescence experiment to check if the circuit works. However, we did not successfully manage to produce GFP from the PAJ cells.

We repeated the fluoresence experiment, using fresh liquid cultures and incubating these cultures with ATC overnight to maximise production of GFP. We successfully proved that the PAJ circuit functions by producing GFP from these cells.
We successfully carried out PCR extraction of the 3 3 kbp fragments.
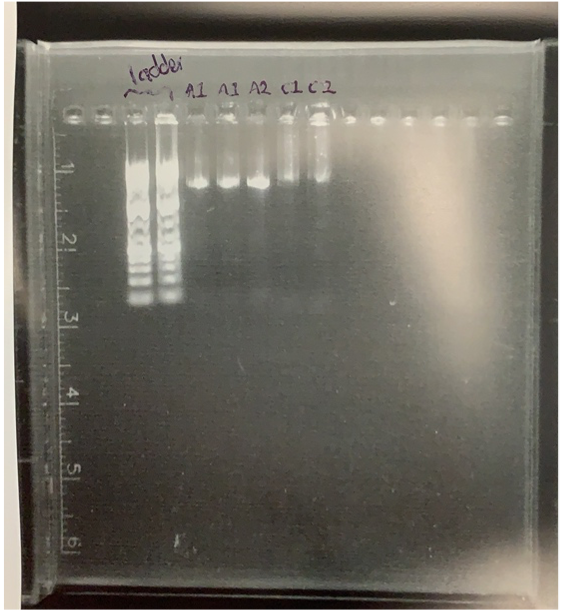
Our attempted purification of the PCR output failed. We realised it was because the PCR had failed so there was nothing to purify.
We carried out PCR and purification again but failed for the second time.
We spent a few days trying to debug the purification procedure and figure out why it wasn’t working. One possibility was that the purification kit we used was contaminated or faulty.
We repeated the PCR for the third time.
We reattempted the purification with a fresh kit and it worked beautifully. We also obtained pure MG1655-Z1 cells.
We attempted assembly of the helper plasmid and transformation into MG1655-Z1 cells.
We found out that yesterday’s work failed.
We reattempted PCR extraction with a new polymerase, allowing us to PCR out the full 9 kb backbone
Purification of the PCR output and assembly seem to have worked according to gel electrophoresis. We also attempted transformation with the helper plasmid.
Our transformation failed. We decided to use more competent cells to purify the assembly output by transformation.
Successful transformation of competent TOP10 E. coli with the helper plasmid allowed to then prepare liquid culture for miniprep.
We miniprepped the helper plasmid then prepared more competent MG1655-Z1 cells.
We transformed our Z1 cells with the helper plasmid.
The transformation was assessed as successful over the weekend. This allowed us to extract backbones for the operator plasmids through PCR.
We realised that a part of SoxS-MCP has not arrived from IDT with the rest of it. However, we still managed to create competent Z1s containing helper plasmids.
The SoxS part from IDT finally arrived. We attempted operator plasmid assembly and transformation.
We discovered that the transformation failed as the DNA was not concentrated enough. We would have to go through more competent cells and miniprep again to repeat, so unfortunately we ran out of time to complete our wet lab work.
The next stage would have been to transform the operator plasmid into our cells and then carry out a fluoresence test to prove that the gRNA and dCas9 system works according to plan and successfully produces a visual indicator in the form of GFP. This would have provided us with real data which we could use to obtain proof of concept. However, we did not have time, which is a shame but we are still proud of all the progress we made in the lab over the summer.