The original idea was to modify a disinfectant spray that would cause bacteria on a surface to fluoresce is they were resistant to the disinfectant. As a result, we contacted and held a meeting with Dr Richard Hastings, the Healthcare Regulatory and Technical Manager at Mirius, a company producing disinfectants and other cleaning solutions. Following this discussion, we decided that a swab-based approach would be preferred over a spray-based one. The main reason was that the concentration of our product, when sprayed onto a large surface, would be unknown and would vary widely. This would lead to varying and unreliable results. Additionally, if we were to integrate our product into a disinfectant spray, there was a high risk that the strong chemicals used in the spray would degrade the proteins and other components that were essential for our system to work. Therefore, we decided to adapt our project to a swab-based approach, which would be more consistent and therefore reliable. After researching and discussing this new approach at one of our weekly meetings, we decided the idea could be further improved upon. Instead of testing for disinfectant resistance, we would test for antibiotic resistance, as this was a much larger issue, and our project could make a greater impact.
As a result, we spoke to a hospital-based geriatrician as a preliminary, non-compulsory scoping exercise to find out some rudimentary opinions. We found out that E. coli and pneumococcus infections are the most common infections that they encounter and lead to a lot of problems. We also spoke to an ITU consultant, who told us a similar story with pneumococcus and Gram-negative infections causing the most issues. As a result, our initial thought was to target Streptococcus pneumoniae. However, this meant we would have had to work in a category 2 laboratory, which would have been difficult to achieve under iGEM safety guidelines. Therefore, following the feedback from our preliminary research, it was suggested that we work with Gram-negative infection (E. coli) instead, as we could do this in a category 1 laboratory. When researching E. coli, we came across CRE as a major issue in a recently published Public Health England report. This report made us decide that our project should be centred around CRE detection.
Following this decision, we went back to ask what we could do to help the situation regarding CRE. Both the ITU consultant and the hospital-based geriatrician we were in contact with suggested that better infection control procedures and teams in hospitals would make the biggest difference. They also told us that amongst all the infections they encountered, approximately 20% of them were hospital acquired infections, an alarmingly high statistic. An A&E nurse informed us hospitals now routinely swab every patient on admission for CRE, but they did not know how long the results take. Subsequent research into this told us that lab results take several days to return a positive result. This exposes hospitals to the risk of potentially unchecked spread of CRE in the meantime. As a direct result, a rapid detection method would help allow infection control procedures, like isolation, to be implemented faster. We also hoped that an easy to carry out test, would encourage infection control teams to be more diligent in swabbing for CRE outside of just in A&E departments. As a result, this method would tackle the previously identified problem with infection control procedures and help decrease hospital acquired infections in general, which we later showed through our computational modelling. As a result, a rapid and easy to use detection method became our goal.
The next stage was to figure out how this rapid detection method would work. We spoke with Dr Freya Harrison, a specialist on Carbapenem resistance, who advised us to look at using phages or peptidoglycan layer tagging. She also taught us a lot about the infectious dose and issues surrounding biofilms. As a result of this meeting, we contacted and met with Dr Antonia Sagona, a phage therapy specialist, about the possibility of using phages in our project. We discovered that this would be too specific for our liking, as it would only allow us to detect one strain of Carbapenem resistant bacteria, which would be of limited use in a hospital. Therefore, we decided to avoid using phages to create our rapid detection method. You can view more detailed information about these meetings on the planning page of our wiki.
While we originally considered designing a faster way of diagnosing CRE in patients, we encountered two problems. After speaking to a palliative medicine consultant and an orthopaedic surgeon, we found out that hospitals nearly always already know what bacteria they are treating. What they do not know is whether said bacteria is resistant to any particular antibiotics. This makes diagnosing CRE as simply an E. coli infection generally an unnecessary task. Therefore, we decided trying to diagnose a bacteria’s level of resistance would be more useful. However, during a meeting with Dr Richard Hastings later that month, he informed us that there are many legal restrictions and regulations surrounding the use of biological products on humans. That meant it would be difficult to design a rapid detection method to diagnose human patients with CRE. It would also take much longer to theoretically get it approved and certified for use in hospitals. As a result, we looked at other ways to detect CRE.

A photo of our meeting with Richard Hastings
Following Dr Hastings’ advice, we decided that containing the spread of CRE by targeting reservoirs like sinks and faucets would have a bigger impact and be more achievable. This would work to prevent multiple patients from becoming infected in the first place rather than just diagnosing one patient’s specific stage of antibiotic resistance. Furthermore, regulations around surface testing are far more relaxed and require far less time to be tested and approved, leading to an earlier potential deployment of our product. As a result, we designed our product to be used to test surfaces. This would seamlessly integrate into current procedures, as any swabs already taken for CRE can also be used with our device. Furthermore, adapting our product for direct patient testing in the future would be technically easy. While patient testing is not the direct focus of our product, we consider it something possibly worth exploring in the future.
Another important consideration was the safety of the contents of our testing kit, to ensure regulatory compliance. After our meeting with Dr Hastings, we were inclined to look further into regulations, to ensure our final product was viable. These inquiries made us realise the use of malachite green as a fluorescence indicator, a strong irritant, in our testing kit was a potential issue. Therefore, we further adapted our product by using a combination of iSpinach, a safer RNA, and harmless fluorophore called DFHBI for the same purpose. The use of living cells in our testing kit also proved a problem. An orthopaedic surgeon we contacted informed us that the risk of contamination meant our product would possibly not get approval for use in hospitals. This led us to investigate the possibility of designing a cell-free system, where living cells would be lysed. Our goal was to design the final product such that it would only contain the necessary components from those cells, with no risk of contamination. In these ways, safety and adherence to regulations were central to the development process.
Considering the feedback from the orthopaedic surgeon and embracing the idea that an easy-to-use test would be most effective, we began to design how our cell-free product would work. We created a prototype for this product’s container, which consisted of a swab, one large reaction tube and two small tubes containing solution 1 and solution 2. A swab would be taken of a surface and placed into the large test tube, where solution 1 could then be added to lyse the cells. Solution 2 would be added after, which, using our engineered CRISPR system, would detect the presence of our target RNA transcribed from the OXA-48 Carbapenem resistance gene. Therefore, if the bacteria present on the swab are resistant to Carbapenems, then the tube will glow green when illuminated by UV light, a very distinct and easy to recognise signal. More information on our designed test kit can be found on the implementation page on our wiki.
In September we designed a survey to assess how aware the general public were of CRE. After gaining ethical approval, the survey was created using Qualtrics and distributed both via email to all SLS undergraduate students at the University of Warwick and via our official Warwick iGEM Instagram and Twitter pages. The survey contained 4 questions, as you can see below.
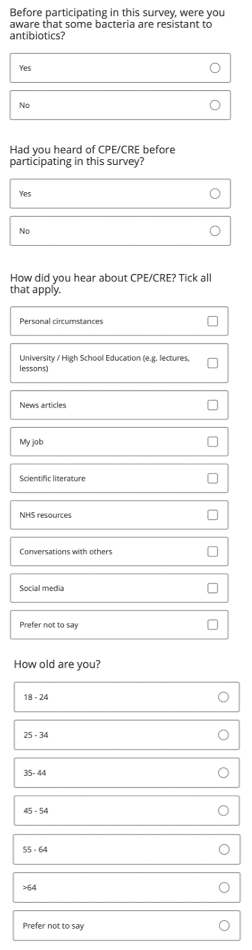
A photo of the survey questions
We managed to obtain 508 responses from a large range of ages, meaning the results should be relatively representative.
A bar chart showing the breakdown of the age ranges of the survey’s respondents
In response to the first question, we found that the majority of the public (91%) were aware that some bacteria are resistant to antibiotics. While this seemed to be relatively common knowledge, when asked about CRE in particular, only 14% had heard of it before participating in our survey.
A pie chart showing the majority of the population are aware of antibiotic resistant bacteria
A pie chart showing the majority of the population haven’t heard of CRE
When split by age, we found that >64s were the most likely to be unaware of CRE, as only 8.45% of those who answered the survey had heard of it before. Those aged between 25 – 34 were marginally more likely to be aware of CRE, but generally there was no particular subset of the population that was overwhelmingly more aware of it. The majority of participants from all ages had not heard of the infection before.
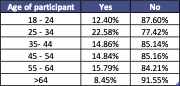
A table showing the age breakdown of whether people are aware of CRE
Next, we looked at the subset of the population who had heard of CRE before participating in our survey and discovered that they were most likely to be aware of it due to their job (37.8%). However, young people aged between 18 and 24 were the least likely to have heard of it this way, as only 7.4% of them selected this option. Most frequently, they had heard of it through University/High School education (40%) and/or scientific literature (18.5%). Participants were least likely to have heard of CRE for the first time from NHS resources, suggesting that there are not many resources out there to help educate the public on this illness. Only those studying a specific degree or in a particular job were likely to be aware of it, which is not representative of the general public.
A bar chart showing the breakdown of how people became aware of CRE
Therefore, since only 14% of the public knew what CRE was, we designed and distributed educational materials to raise awareness of CRE and inform people of how our project might make an impact on the spread of this disease. For example, we created this poster to provide an introduction into what CRE is, how we can detect it, and how to reduce its spread, as we have seen that NHS resources are not adequate for accomplishing this task.
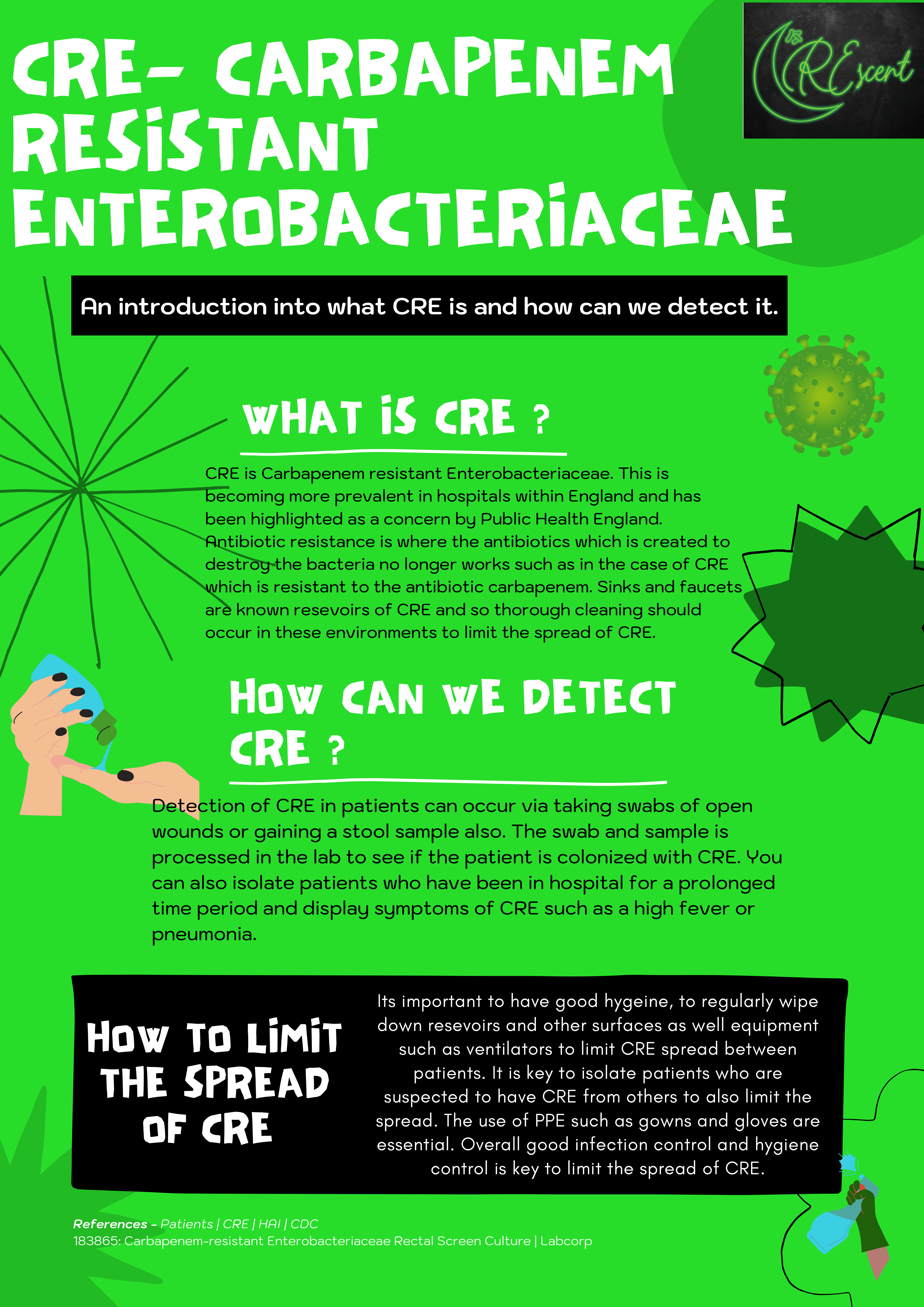
A poster we produced to spread awareness about CRE
We then posted the materials we created onto our social media to distribute it to members of the general public.

A social media post of our poster
More materials can be found on the education and communication page of our Wiki
During this time, we were developing a model to try to show how effective our solution could be if deployed. However, up until this point the model did not show our product having much of an effect on the outcome of our simulations. There seemed to be little change in the number of patients who acquired pathogens resistant to Carbapenems or patients that died. This was due to a fault in the logic in the initial design of the model. The issue was that the model put people into isolation if they were being treated with Carbapenems. However, that implies the hospital does not believe the patient can be resistant against Carbapenems, as then Carbapenem treatment would be ineffective. This was therefore not only unrealistic (only Carbapenem resistance should have been identified as an issue worthy notable enough to require isolation), but meant our product was barely useful. The product only detects people with Carbapenem resistance, which requires a patient to be treated with Carbapenems for it to develop. Therefore, the product had little effect, as almost everyone it could identify was already in isolation.
As a result, we reached out to Dr Alexander Darlington, who suggested that we include an additional last-resort drug (e.g. Colistin), which would allow patients treated with Colistin to be placed into isolation rather than those treated with Carbapenems. Therefore, we adapted the model and made the appropriate changes, meaning a more realistic number of people were put into isolation while allowing our product to be useful. You can view the final model on the model page of our Wiki
We hope that our project will be used in the future to not only reduce the spread of CRE in hospitals, but also all hospital acquired infections in general. We designed our system is easily adaptable to different types of antibiotic resistant bacteria (e.g., pneumococcus, which we wanted to target but couldn’t), by simply changing the gRNA to target a different gene. We also hope that our system might be able to be used to directly diagnose patients with CRE so they can receive the correct treatment as fast as possible to reduce the severity of the disease and the risk of death. We have designed a prototype for our rapid test kit, and hope that this will be adopted by hospitals in the future to help detect CRE on surfaces or in patients, depending on what happens with the technology we have designed.
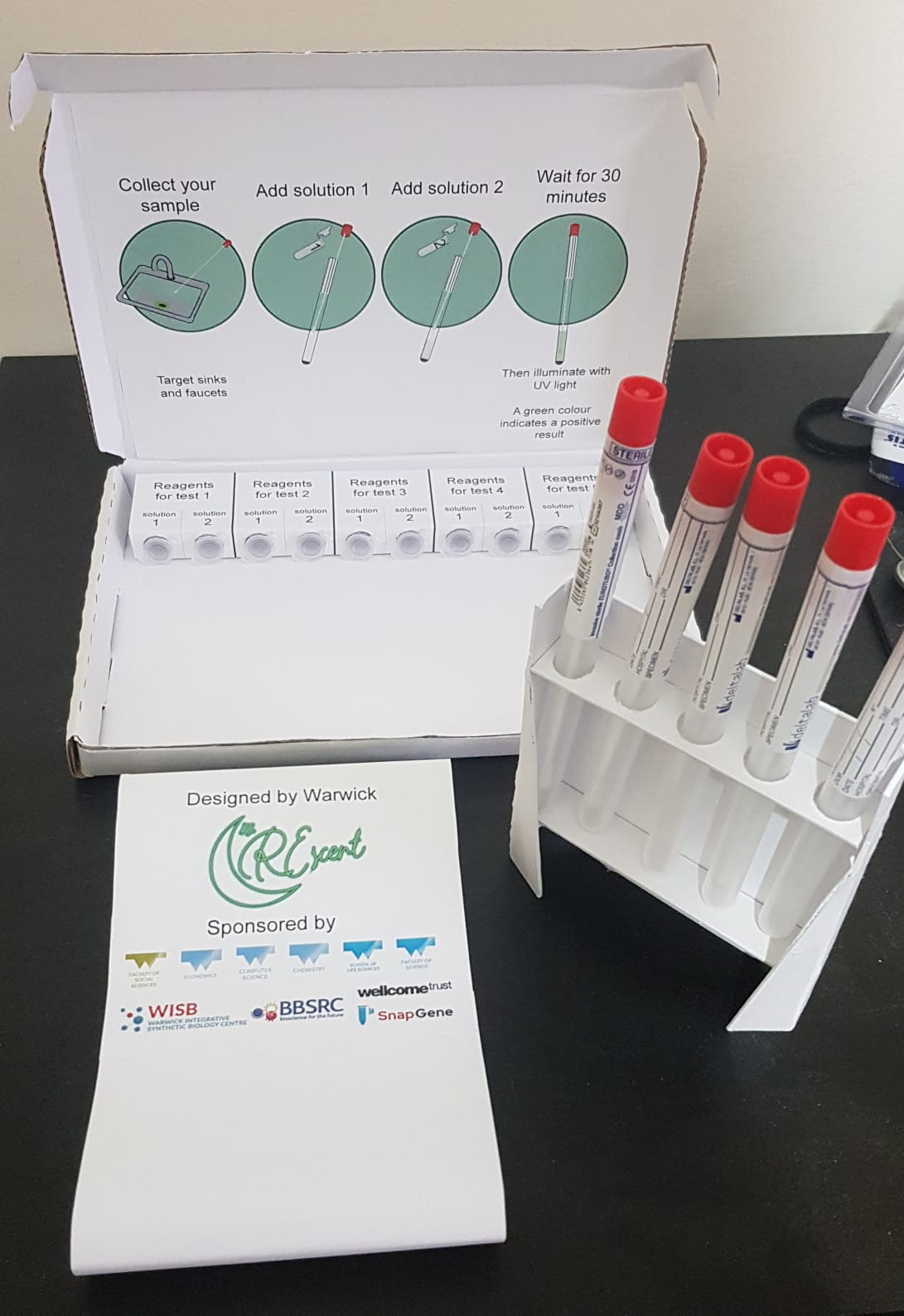
A photo of the prototype test kit